Point-of-Care Testing
The arrival of next generation advanced diagnostic point-of-care tests is here. True Diagnostics goes beyond the traditional clinic setting and provides access to lab-quality diagnostics in even the most remote settings. The TrueDX PlatformTM enhances the ease of use and economics of scale over conventional diagnostic technologies, thereby allowing for greater market opportunities. True Diagnostics, Inc. were able to open up new market opportunities in emerging markets, including in the People’s Republic of China, leveraging the TrueDX PlatformTM.
QuikPacII™ is the New Rapid Test That Provides Accurate Immune Response to COVID-19 Infection Diagnosis in 15 Minutes.
It is widely accepted that IgM provides the first line of defense during viral infections, followed by the generation of adaptive, high affinity IgG responses for long term immunity and immunological memory. Testing of COVID-19 IgM and IgG antibodies is an effective method for the rapid diagnosis of Immune Response to COVID-19 infection. Detection of COVID-19 IgM antibodies to indicate a recent exposure to COVID-19, whereas detection of COVID-19 IgG antibodies indicates a later stage of infection. Thus, this combined antibody test could also provide information on the stage of immune response to infection.
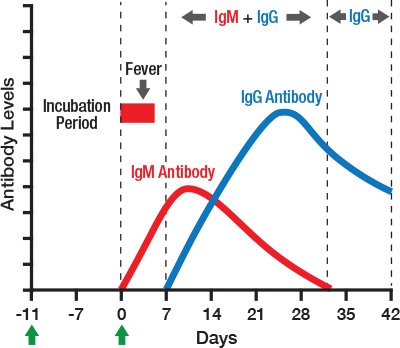

It can be used for rapid screening of carriers of the virus that are symptomatic or asymptomatic. Recent studies suggest that a high percentage of patients show no clinical symptoms of the virus, thus screening patients is vitally important. The test is ideally suited for hospitals, clinics and test laboratories. The test can also be effectively deployed in businesses, schools, airports, seaports and train stations, etc., giving it the potential to become a compelling force in the fight against this global threat.
The test was validated using 602 clinically positive and negative patient samples. The overall test sensitivity of QuikPacII™ COVID-19 IgG/IgM is 89.4% and specificity is 97.7%. • For Rx use only. • This test has not been reviewed by the FDA. For use in clinical laboratories by health care professionals following FDA guidance “Policy for Diagnostic Tests for Coronavirus Disease-2019 (COVID-19) during the Public Health Emergency”. • Negative results do not rule out SARS-CoV-2 infection, particularly in those who have been in contact with the virus. Follow-up testing with a molecular diagnostic assay should be considered to rule out infection in these individuals. • Results from antibody testing should not be used as the sole basis to diagnose or exclude SARS-CoV-2 infection or to inform infection status. • Positive results may be due to past or present infection with non-SARS-CoV-2 coronavirus strains, such as coronavirus HKU1, NL63, OC43, or 229E. • Not for the screening of donated blood. • For Export Use Only





